The Pharmaceutical Quality System (PQS) is a comprehensive management framework designed to ensure that a pharmaceutical company consistently produces high-quality products. Based on the principles of ICH Q10 and aligned with ISO 9000 standards, the PQS is essential to ensure the quality and efficacy of medicinal products, safeguard patient safety, and enhance overall business performance. At its core, a PQS is built around a quality strategy that spans the entire life cycle of a pharmaceutical product, focusing on achieving these critical objectives.
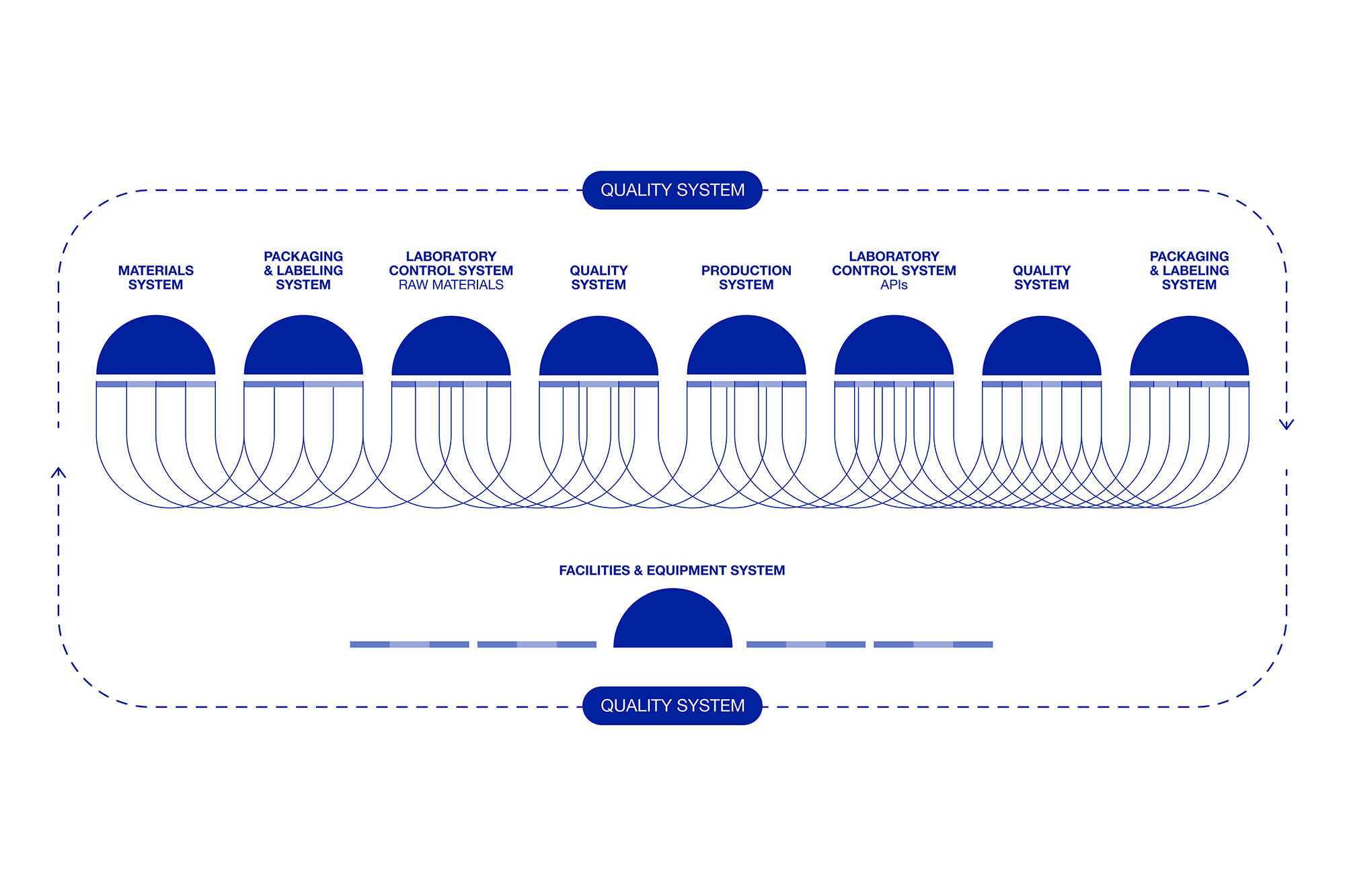
The PQS is built on six interconnected systems that work together to maintain and improve quality. These systems are foundational to the production of Active Pharmaceutical Ingredients (APIs) and other pharmaceutical products. They interact closely to manage quality risks effectively and to support continuous improvement.
In the pharmaceutical industry, the pursuit of innovation must be balanced with strict regulatory requirements, particularly those related to patient safety. The PQS is designed to manage this balance by integrating robust quality risk management practices. This approach helps reduce quality risks while fostering innovation within a highly regulated environment.
According to ICH Q10, a robust PQS is built on four essential elements, each designed to be applied consistently throughout the product’s life cycle:
Performance Monitoring System: This system continuously monitors process performance and product quality, ensuring control and identifying areas for potential improvement.
Corrective and Preventive Actions (CAPA): Triggered by quality indicators and trend monitoring, the CAPA system identifies root causes of issues and implements corrective measures to prevent recurrence.
Change Management System: Whether driven by surveillance, CAPA, or other improvements, managing change is crucial to fostering innovation. Quality by Design (QbD) principles, when applied, allow for greater regulatory flexibility in handling these changes.
Management Review: Regular reviews of process performance and product quality provide an ongoing assessment of the PQS’s adequacy and effectiveness, helping to fine-tune the system for optimal outcomes.
Together, these elements create a dynamic framework for managing the PQS and fostering continuous improvement in both products and services. These elements of the PQS are not static; they are constantly evolving tools that drive continuous improvement in both product quality and overall operational efficiency. By integrating these principles, pharmaceutical companies can enhance product quality, reduce risks, and maintain a strong focus on patient safety.

Quality at Seratec plays a crucial, cross-functional role. For us, quality isn’t just a goal—it’s a driving force behind everything we do.

Seratec specializes in areas ranging from developing active molecules to offering turnkey solutions for APIs.
The FDA ensures all pharmaceutical products, including APIs, meet top quality and safety standards.