Entrepreneurs in pharmaceutical chemistry since 1970, we produce active pharmaceutical ingredients (APIs) for the treatment of rare and orphan diseases. Backed by internationally recognised French expertise, we design, develop, produce and register dossiers for pharmacologically active molecules with the US (FDA), European (EMA) and European Medicines Agency (EMEA).
(FDA), European Medicines Agency (EMA) and national medicines agency (ANSM). To offer the best possible service, we also produce APIs in accordance with quality standards known as Good Manufacturing Practice (GMP or cGMP). The pharmaceutical companies we work with are very demanding. Because the battle they are waging is just as demanding.
Company values:
Commitment, Flexibility and Passion
Quality Control laboratory
The site’s Quality Control laboratory is responsible for carrying out analyses of raw materials, in-process products, finished products and stabilities in accordance with pharmacopoeial standards (USP, European, etc.), as well as cleaning controls and analyses for R&D purposes.
The laboratory also develops and fully validates analytical methods, with the aim of submitting methods for regulatory approval to the American and/or European authorities.
The laboratory is equipped with a range of basic physico-chemical analysis equipment: pH, conductivity meter, melting point, oven, drying oven, etc., as well as chromatographic equipment:
HPLC, CPG and CPI. The laboratory has a high level of quality, particularly in terms of data integrity, as demonstrated by the use of a managed and validated client server for chromatographic data.
Location: Courville (28)
Type of contract: Fixed-term contract
Start date: as soon as possible
Hours: Fulltime

The Pharmaceutical Quality System (PQS) is a management framework ensuring consistent production of high-quality products.
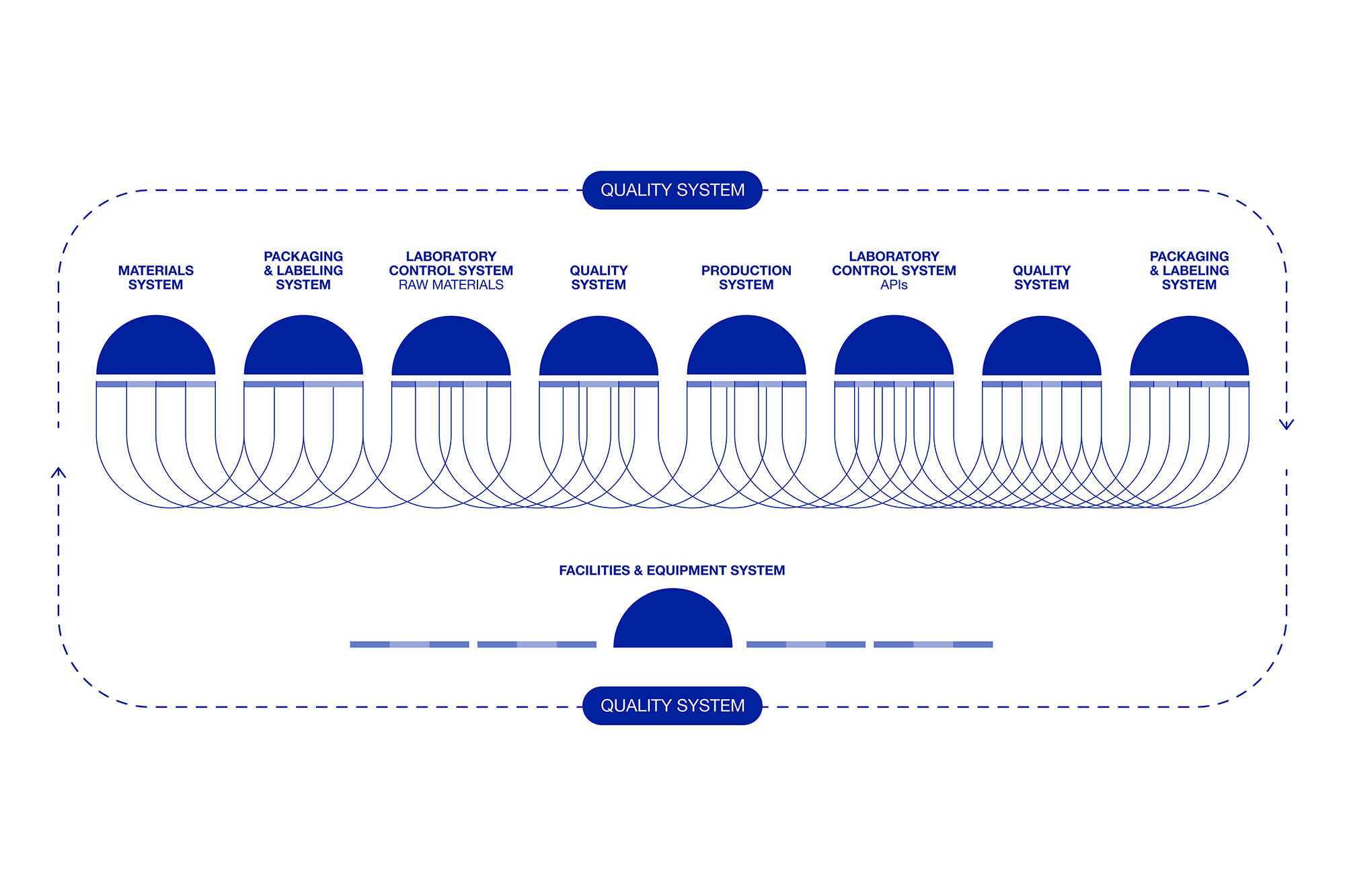
The FDA ensures all pharmaceutical products, including APIs, meet top quality and safety standards.

At Seratec, quality is not just a goal—it’s the foundation of everything we do and embedded throughout the entire product life cycle.